The project
We developed our client’s initial concept for a novel medical surgical device into a working prototype for demonstrating to investors to secure future funding.
The challenge
Our client’s early work had produced promising results but they needed support designing a more complex multichannel prototype and compiling a draft technical file for CE marking. CircuitWorx’s experience in product development and our expertise in electronics hardware design, embedded firmware and PC software were exactly what was needed to advance this project.
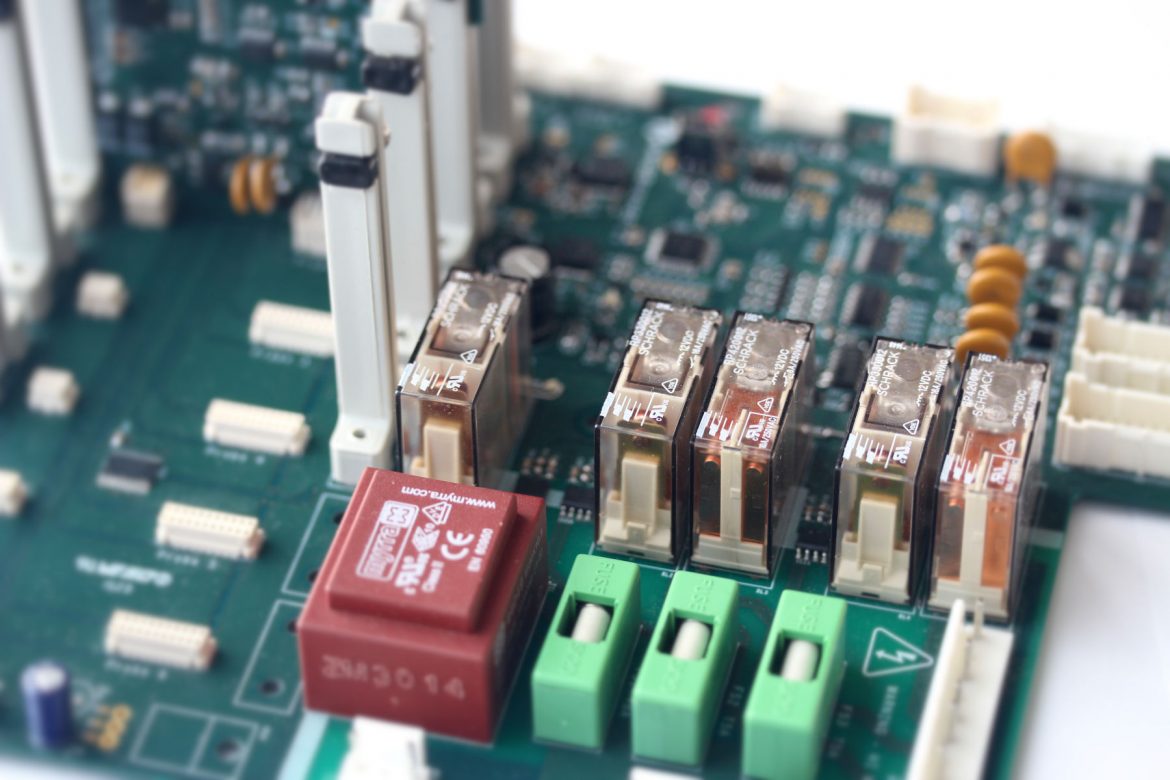
Through interpretation of legacy documents, structured conversations and careful consideration of risks we guided our client in the production of a Device Hazard Analysis and Product Requirements Specification. From this we developed the details of the electronics hardware and software requirements and architecture, designed the circuits, laid out PCBs, designed and coded the software, and built and tested the boards. We produced Verification Protocols for each of our sub-systems with full traceability throughout to ensure all the specified requirements had been fulfilled.
Throughout the product lifecycle we maintained a close collaborative relationship with other third-party developers (industrial & mechanical designers, component manufacturers) to ensure an integrated system design.

The CircuitWorx touch
Sometimes, you don’t always know exactly what you need – so we’ll always offer helpful suggestions when we can. For this client we developed some additional PC software which connects to the device via USB; this allowed them to see the exact outputs of various sensors and adjust settings in situ in real-time.
We were also pleased to support this client with a number of on-site visits to provide calm and reassuring advice and guidance through the often-stressful system integration and verification phases of the project.